- Date: Jun 09, 2020
- Category:
- Use of Wearable Devices in Clinical Research and Trial
- Extensive Use of Smart Medical Devices
- Development of Medical Devices to Treat Opioid Addiction
- Strengthening of FDA’s 510(k) Program to Ensure Device Safety
- Increasing Surveillance and Postmarket Data Collection of Medical Devices
- Innovative Devices for Less Invasive Heart Surgery
- Final Thoughts
The medical devices and equipment industry have experienced significant changes within the past few years. From regulatory updates to technological innovations, there has been quite a lot happening, and such regulatory changes and trend shifts are likely to continue even in 2020 as well.
Medical devices are an essential resource for the entire healthcare industry. As chronic illness and serious health issues keep the medical landscape worried, it is through innovative and advanced devices that healthcare providers can keep up their promise of delivering timely and quality care to an increasing population worldwide. While trends keep coming and going, here are the five key trends that are likely to bring massive transformation in the medical device market. Have a look:
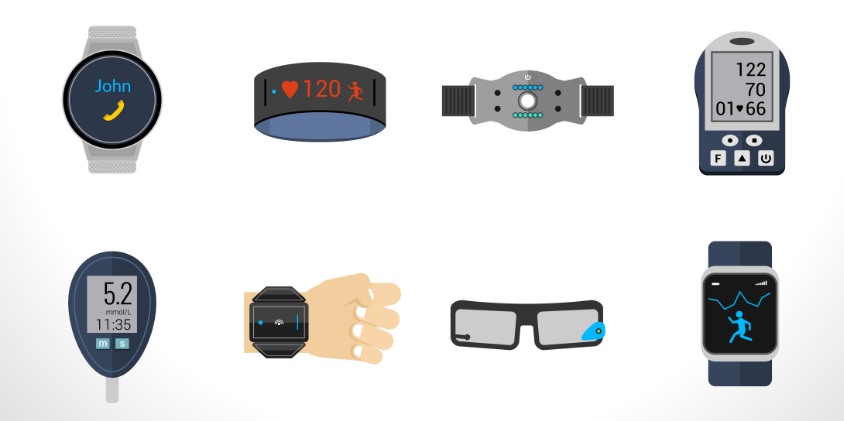
Use of Wearable Devices in Clinical Research and Trial
An increasing number of health-conscious people are found using wearable devices to track, monitor, and measure their vital stats, calorie loss, etc. There are more than 325 million who own connected devices and wearables globally. Whereas there are more than 2.5 billion people, who own smartphones. These devices not only impact the personal life of users but are increasingly being used across various industries at a professional level for many purposes.
One such area where wearables are used is the field of clinical trial and research. Wearables and connected devices are being used to collect data during clinical trials. Patient-generated data fetched through these devices are easy and faster to obtain. This helps researchers to better monitor patients and identify potential health threats to address them on time and prevent any adverse situation. Hence, a lot of clinical researchers are incorporating wearables into their care delivery models to collect patient data so that they can improve the quality of care. However, using wearables and connected devices have their share of challenges. Especially in the healthcare field, the regulations and guidelines for collecting and managing sensitive patient data are stringent. While using devices to collect insights, researchers need to ensure that they follow the guidelines and comply with the regulatory policies. They also need to strategize and organize the procedure to collect and store data when it comes from multiple sources.
Extensive Use of Smart Medical Devices
The healthcare sector has witnessed a massive breakthroughs in terms of innovation and technological advancement. From natural language processing to Artificial Intelligence (AI) backed telehealth services, automated medical billing, machine learning, and cognitive computing, every element of the age of the smart machine has penetrated the healthcare industry, making way for improved care.
A variety of smart applications and medical devices are being used to diagnose, monitor, and offer therapeutic and preventive treatments to patients. Today, people use a toothbrush with sensors, AI-powered insulin pumps, smart asthma monitoring devices, and even dentists to use smart drills for oral treatment. Many companies are coming up with smart medical devices that are aimed at offering timely care to patients. Started a few years back, the impact of smart and connected devices has been quite prominent even in 2019.
From smart socks for skin inflammation detection to portable visor that detects strokes by sending low-energy radio waves through the brain, the use of smart devices for medical purposes is widespread. That is why it is estimated that by 2024, the global smart medical device industry will reach $64 billion, as per Zion Market Research.
Development of Medical Devices to Treat Opioid Addiction
One of the most serious public health issue or crisis in the US is that of opioid misuse, abuse, and addiction. As per records in 2017, there were more than 72,000 Americans who died because of a drug overdose. Opioids are substances present in certain prescribed pain medications and illegal drugs like heroin. Often, opioids are given to relieve pain, but with prolonged intake body may become dependent, and the patient may get addicted to it. Consuming more than the prescribed quantity of opioids may result in death.
That is why last May, the Food and Drug Administration (FDA) came up with the innovation challenge to boost the development of diagnostic tests, digital health technologies, and medical devices that can combat the opioid crisis with solutions that help in detecting, preventing, and treating opioid addiction. Out of the 250 applications submitted, FDA chose eight developers and their innovative approaches. Here are some of the suggested medical devices with the potential to work towards delivering the best solution for the life killing addiction:
- Smart pill dispenser
- Rapid drug screen
- Virtual reality neuropsychological therapy
- Transcranial magnetic stimulation (DTMS) device
Strengthening of FDA’s 510(k) Program to Ensure Device Safety
As per FDA’s Section 510(k) of the Food, Drug, and Cosmetic Act, medical device manufacturers require to register their intent at least 90 days in advance of marketing a medical device. This particular procedure is called Premarket Notification or 510(k) or PMN. They need to submit premarket notification before launching a new device into the commercial market or reintroducing an existing device with modifications such as changes related to the energy source, material, design, chemical composition, intended use, or manufacturing process. The purpose of having this special 510(k) program is to ensure patients get timely access to the latest medical devices that are safe, high-quality, and effective. To comply with the guidelines of the program, manufacturers need to leverage efficient review practices to ensure their medical devices are appropriate.
Recently, the FDA introduced a new action plan known as The Medical Device Safety Action Plan: Protecting Patients and Promoting Public Health. Under this plan, further changes have been introduced in the existing regulations, which include increased documentation for 510(k) submission and increased review time. This is done to strengthen the 510(k) program that allows new medical devices, which are substantially equal to existing devices, to be exempted from the procedure of premarket approval.
Increasing Surveillance and Postmarket Data Collection of Medical Devices
One of the key aspects of this year’s medical device industry has been FDA’s constant efforts to strengthen the medical devices market with quality and safety checks in place. An increasing priority has been given to postmarket data collection of medical devices and improved surveillance initiatives. Here are a few steps that the FDA have taken or are soon going to implement for the purpose include:
- Establishment of a national system for collecting real-time evidence and seeking additional government funding of $46 million for the same.
- Gathering of clinical data from medical devices used in women’s health, such as vaginal rejuvenation devices, breast implants, etc.
- Using real-world evidence, the FDA is planning to conduct test case demonstration projects. For instance, undertaking projects to compare the effectiveness and safety of different tissue closure techniques.
- Besides the Medical Device Reporting regulation 21 CFR 803, all manufacturers will have to follow more stringent regulations of the European Medical Device Regulation (MDR) if they sell devices in the Europe region. Published in 2017, the MDR implementation is going to be in May 2020, and all manufacturers will have to comply with it.
Moreover, clinical researchers will also require to comply with their data collection and review process in compliance with the new European MDR regulations.
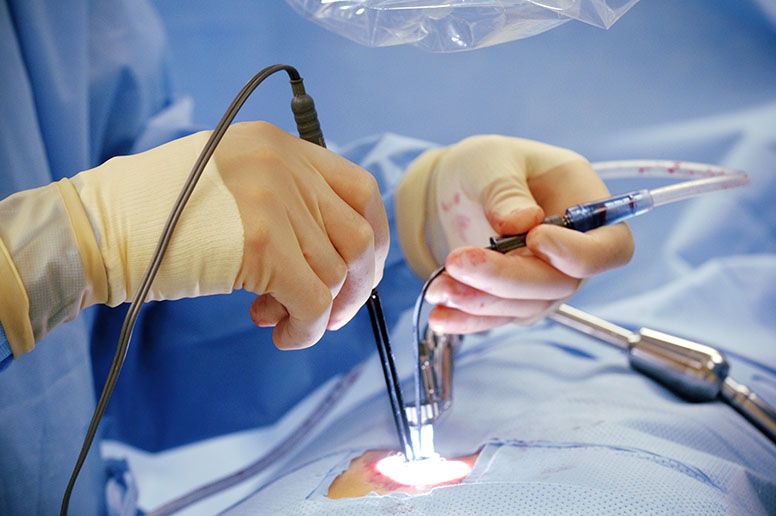
Innovative Devices for Less Invasive Heart Surgery
Thanks to the technological disruption that the medical device industry has been witnessing major innovations in recent years, and the trend is most likely to continue in the future as well. Especially in the surgical space, advanced medical devices are making it possible for surgeons to conduct less invasive surgeries that not only save a life but also reduce recovery time and possibilities of hospital re-admissions.
With this less invasive approach in execution, medical devices companies are coming up with innovative technologies and devices that can improve the quality of care. AN increasing number of heart surgeries are being performed through a catheter in the skin, which is commonly known as the mitral and tricuspid valve percutaneous replacement surgery. The practical implementation of less invasive surgeries that require minimal cuts is slowly increasing in numbers. Now, even medical device companies are planning to introduce the first transcatheter mitral valve replacement device as part of further improvement. Similar efforts are likely to be more in the days to come for sure.
Final Thoughts
With a market size of over $156 billion, the United States medical device market remains the largest in the world, and the numbers are likely to increase by 2023, reaching $208 billion. In recent years, not only revenue-wise, even innovation and development-wise, the medical device market has seen an immense upsurge. Connected and wearable devices have made their way into almost every healthcare domain. In fact, many leading Medtech companies are keen on experimenting and have already started introducing new technologies such as robotic-assisted surgery. Skilled surgeons are using surgical robots to perform operations with minimal incisions.
With every passing year, the medical device market is witnessing new trends and approaches that aim to improve care services through efficient medical devices. Even the FDA is unveiling better regulations and taking steps to ensure faster approval of safe medical devices that can contribute towards the growth of the medical device and equipment industry.